Ferromagnetism: Difference between revisions
No edit summary |
No edit summary |
||
| (3 intermediate revisions by one other user not shown) | |||
| Line 1: | Line 1: | ||
== What is ferromagnetism? == | == What is ferromagnetism? == | ||
Ferromagnetism is the type of magnetism exhibited by iron, nickel, and cobalt. The effect in iron is much stronger than in nickel or cobalt. Compared to paramagnetism and diamagnetism, ferrormagnetism is several of orders of magnitude stronger. Ferromagnetic materials are also able to retain magnetization outside of an external current or magnetic field. This is because iron and other ferromagnetic materials naturally form "magnetic domains," where a large group of atoms (in the billions) naturally align to each other to create an area with a net magnetic field. There are millions of these magnetic domains, and their magnetic moments are randomly aligned so that your average piece of iron is magnetically neutral. Otherwise any old piece of iron would be an incredibly strong magnet. In the presence of an external magnetic field, the domain boundaries shift, such that domains with magnetic fields in the same direction as the external field grow and gain more atoms from neighboring domains that weren't aligned with the field. This boundary shift is caused by the realignment of atoms on the domain boundaries to try to line up with the external field. The strength of the external field determines the extent of this reordering and thus the strength of the ferromagnetic reaction. One interesting characteristic of ferromagnetism is that when a ferromagnetic material is heated to a certain point, the domain boundaries cease to exist and the atoms align randomly as they have too much energy to remain in the domains. As a result of the random alignment of atoms, all ferromagnetic effects cease above this temperature. The temperature where this occurs is called the Curie temperature. This effect can be exploited to create strong magnets by heating a ferromagnetic material to above this temperature and then cooling it in the presence of a strong magnetic field. The external field will ensure that a majority of the domains that form will be aligned with this field, giving the material a permanent net magnetization. (Griffiths, David J. 1999. ''Introduction to Electrodynamics: Third Edition''. Prentice-Hall.) | Ferromagnetism is the type of magnetism exhibited by iron, nickel, and cobalt. The effect in iron is much stronger than in nickel or cobalt. Compared to paramagnetism and diamagnetism (covered in the next section), ferrormagnetism is several of orders of magnitude stronger. Ferromagnetic materials are also able to retain magnetization outside of an external current or magnetic field. This is because iron and other ferromagnetic materials naturally form "magnetic domains," where a large group of atoms (in the billions) naturally align to each other to create an area with a net magnetic field. There are millions of these magnetic domains, and their magnetic moments are randomly aligned so that your average piece of iron is magnetically neutral. Otherwise any old piece of iron would be an incredibly strong magnet. In the presence of an external magnetic field, the domain boundaries shift, such that domains with magnetic fields in the same direction as the external field grow and gain more atoms from neighboring domains that weren't aligned with the field. This boundary shift is caused by the realignment of atoms on the domain boundaries to try to line up with the external field. The strength of the external field determines the extent of this reordering and thus the strength of the ferromagnetic reaction. One interesting characteristic of ferromagnetism is that when a ferromagnetic material is heated to a certain point, the domain boundaries cease to exist and the atoms align randomly as they have too much energy to remain in the domains. As a result of the random alignment of atoms, all ferromagnetic effects cease above this temperature. The temperature where this occurs is called the Curie temperature. This effect can be exploited to create strong magnets by heating a ferromagnetic material to above this temperature and then cooling it in the presence of a strong magnetic field. The external field will ensure that a majority of the domains that form will be aligned with this field, giving the material a permanent net magnetization. (Griffiths, David J. 1999. ''Introduction to Electrodynamics: Third Edition''. Prentice-Hall.) | ||
== What causes ferromagnetism? == | == What causes ferromagnetism? == | ||
| Line 28: | Line 28: | ||
''Authors: Eric Clay, Jodi Hodge, and David Robbins'' | ''Authors: Eric Clay, Jodi Hodge, and David Robbins'' | ||
''Reviewers: Chris Lau'' | ''Reviewers: Chris Lau'' | ||
Latest revision as of 13:52, 11 January 2010
What is ferromagnetism?
Ferromagnetism is the type of magnetism exhibited by iron, nickel, and cobalt. The effect in iron is much stronger than in nickel or cobalt. Compared to paramagnetism and diamagnetism (covered in the next section), ferrormagnetism is several of orders of magnitude stronger. Ferromagnetic materials are also able to retain magnetization outside of an external current or magnetic field. This is because iron and other ferromagnetic materials naturally form "magnetic domains," where a large group of atoms (in the billions) naturally align to each other to create an area with a net magnetic field. There are millions of these magnetic domains, and their magnetic moments are randomly aligned so that your average piece of iron is magnetically neutral. Otherwise any old piece of iron would be an incredibly strong magnet. In the presence of an external magnetic field, the domain boundaries shift, such that domains with magnetic fields in the same direction as the external field grow and gain more atoms from neighboring domains that weren't aligned with the field. This boundary shift is caused by the realignment of atoms on the domain boundaries to try to line up with the external field. The strength of the external field determines the extent of this reordering and thus the strength of the ferromagnetic reaction. One interesting characteristic of ferromagnetism is that when a ferromagnetic material is heated to a certain point, the domain boundaries cease to exist and the atoms align randomly as they have too much energy to remain in the domains. As a result of the random alignment of atoms, all ferromagnetic effects cease above this temperature. The temperature where this occurs is called the Curie temperature. This effect can be exploited to create strong magnets by heating a ferromagnetic material to above this temperature and then cooling it in the presence of a strong magnetic field. The external field will ensure that a majority of the domains that form will be aligned with this field, giving the material a permanent net magnetization. (Griffiths, David J. 1999. Introduction to Electrodynamics: Third Edition. Prentice-Hall.)
What causes ferromagnetism?
The magnetic domains that cause ferromagnetism are regions in which the spins of large numbers of unpaired electrons of neighboring atoms align with each other, creating a unidirectional magnetic field. This alignment of spins arises from an atomic-level quantum mechanical interaction.
In iron, for example, each atom has four unpaired electrons whose spins align thanks to this quantum interaction. The magnetic force from these combined spins acts upon adjacent iron atoms, causing them to align and propagate the field, adding to its strength. This occurs throughout the iron specimen, giving rise to millions of domains. If these domains are formed in a in the presence of a strong magnetic field under the Curie temperature, the domains will form in alignment, creating a permanent magnet. This is how those ceramic refrigerator magnets are formed.
Two other types of magnetism exist: paramagnetism and diamagnetism. Paramagnetism arises from the individual spins of electrons within atoms. Usually, the spins cancel each other out, but when they don’t, they can produce a small net magnetic effect. When a paramagnetic material, such as water, is exposed to a strong enough magnetic field, it will be attracted to the field. Diamagnetism comes from the electrons orbiting each atom in a material, and it has the effect of opposing any magnetic field applied to it, according to Lenz's Law. All neutral atoms can produce a diamagnetic effect, so nearly all materials are diamagnetic. The reason these two types of magnetism aren’t usually noticed is that they rely on interactions within a single atom. Ferromagnetism, however, uses the interaction of billions of atoms to create a much stronger force.
This section is based on information from the following websites on mechanisms of magnetism:[[1]][[2]]
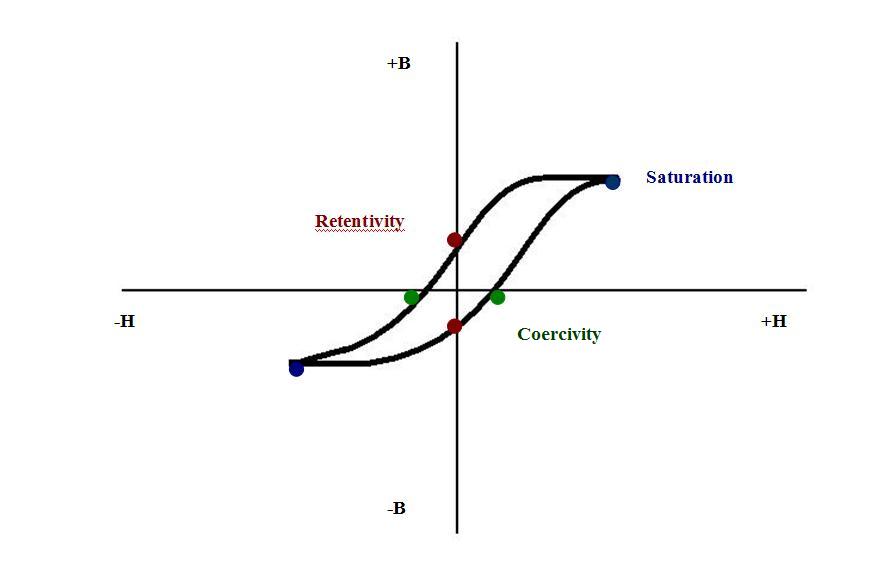
Ferromagnetic Hysteresis Loop
The plot of magnetic flux density (B) versus magnetic flux intensity (H) demonstrates the memory behavior of a ferromagnetic material. If the material is first demagnetized, applying an increasing external magnetic flux causes its domains to rapidly align; the alignment rate slowly decreases until most of the domains are aligned. The point where most of the domains are aligned in one direction and the magnetic flux has created maximum magnetic density is called the saturation point of the ferromagnetic material. At the saturation point, lowering the magnetic flux intensity decreases B, and it decreases faster as it approaching zero magnetic flux. Magnetic memory is described by article "Hysteresis Loop and Magnetic Properties"[[3]] from ndt-ed.org, an educational website on the Nondestructive Testing discipline. When the external magnetic flux is completely turned off, the point on the curve is the retentivity value. The retentivity value is the amount of natural magnetic flux a ferromagnetic material possesses(ndt-ed). Then, further decrease of the magnetic flux to the point on the curve where the strength of material’s magnetic flux density is zero or where all the domains have lost almost all alignment, this point of the material memory is called the coercivity point(ndt-ed). The amount of energy it takes to remove the natural magnetic flux of a magnetic material is called the coercivity force(ndt-ed).
Further increasing the magnetic flux intensity in the negative direction will soon result in most of the domains to align again to a maximum amount,this point is called also called saturation. However, at this saturation point most domains are pointed in the opposite direction than the saturation first experienced in the hysteresis loop. When the magnetic flux intensity is turned off after experiencing saturation in the negative domain, the ferromagnetic material will return to a retentivity value, or the point of intersection on the B axis. The BH curve shows that increasing positive magnetic flux intensity again causes the material to approach positive saturation again. Now instead of zero density located at the origin, as the demagnetized material did, it requires more energy to drive the material to zero density and zero density is now at a higher value of intensity. Changing the negative and positive magnetic flux intensity applied to the ferromagnetic material results in loss and gain of its magnetic density. The area within the BH curve relates to the amount of energy needed to change the behavior of the magnet. Permanent magnets have large, square-like hysteresis curves because it is requires a lot of energy to demagnetize the material. On the other hand, a transformer magnetic properties can be easily changed and its hysteresis curves has little area.
Webpages on the different types of magnetism and their mechanisms:
[[4]]
[[5]]
Authors: Eric Clay, Jodi Hodge, and David Robbins
Reviewers: Chris Lau